NEW PULMONARY FIBROSIS TREATMENT
ON 15 JULY 2019.
Latest News: FDA NDA Submission
New Treatment for Pulmonary Fibrosis (IPF) to Improve Patient Quality of Life
EmphyCorp is proud to announce the completion of a clinical trial to define medical endpoints as requested by the FDA for the NDA marketing application in patients with Pulmonary Fibrosis, under its Orphan Drug Designations for the treatment of Interstitial Lung Diseases (ILD), which includes Pulmonary Fibrosis and Cystic Fibrosis.
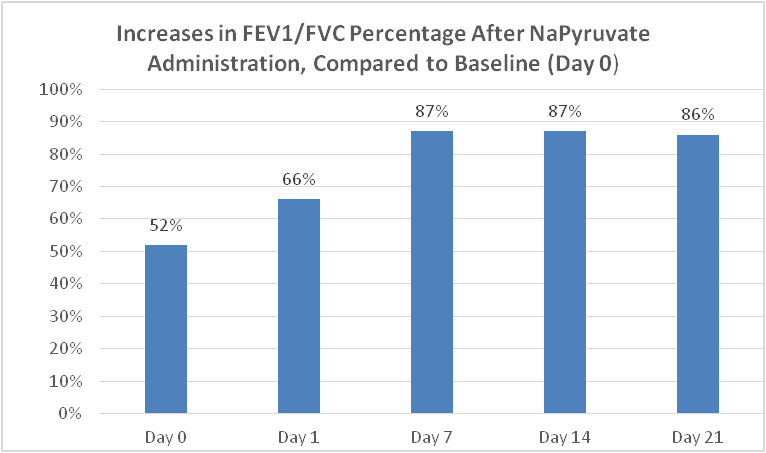
The Clinical trials with our nasal spray demonstrated a statistically and clinically significant increase FEV-1, SaO2, FVC, FEV-1/FVC ratios (52% to 86%),and nitric oxide, (needed to increase bronchial dilation and fight infections), and a significant reduction of coughing, nasal erythema and inflammation and congestion, in all patients tested with Idiopathic pulmonary fibrosis. In all patients, the test results were compared to their previous three-week screening and baseline data (there current therapies) as the placebo control for each variable including all their lung functions, FEV-1, FVC, PEF, FEV-1/FVC ratios, SaO2, Nitric oxide, coughing rates, nasal inflammation.
The data from these clinical studies showed that coughing was significantly (p=0.007) reduced in all patients; a significant (p=0.010) improvement in nasal irritation/erythema with most patients being free of irritation by day 22 (p=0.000); and a significant (p=0.010) increase in the group average expelled-NO by day 8. Unexpectedly a significant (p=0.010) improvement in lung function (breathing) was observed in all patients with Idiopathic Pulmonary Fibrosis by day eight, increasing to p=0.0005 by day 22 compared to baseline, as determined by changes in FVC, FEV1, PEF, and FEV1:FVC ratios.
The improved FEV1/FVC ratio from 52% to 86% was clinically significant and indicated that current therapies in use are inadequate to treat patient with Idiopathic Pulmonary Fibrosis.
Interstitial Lung Diseases, encompass a large group of chronic lung disorders, including Pulmonary Fibrosis, associated with excessive tissue remodeling, scarring, fibrosis, decreased FEV-1/FVC values, decreases in nasal nitric oxide, and a decrease in total lung capacity. 96.88% of Pulmonary Fibrosis patients have congestion caused by nasal inflammation by age 60, which decreases the ability of nasal nitric oxide to maintain normal lung functions leading to increased lung and nasal infections, a reduced SaO2 level, post nasal drip that causes coughing, mouth breathing, and reduced FVC levels.
Nasal steroids and other OTC nasal treatments shut down the synthesis of nasal nitric oxide, which leads to decreased lung function and a 34% increase in infections. There were 32,381 complaints to the FDA that nasal steroidal products and all the over-the-counter products have failed to provide efficacy in the relief of nasal inflammation and have failed to increas lung function in patients with Pulmonary Fibrosis.
EmphyCorp globally patented nasal delivery system, with sodium pyruvate which is a naturally occurring antioxidant of the human body, has been shown to significantly reduce inflammatory agents throughout the human body, including the lungs as demonstrated in eight Phase I/II and Partial Phase III human studies and eight human nasal studies submitted to the FDA in patients with, COPD, Pulmonary fibrosis, and CF patients for our NDA submission. Over one and a half million nasal spray units have been used to treat patients in over 100 hospitals, including an estimated 150,000 patients with COPD, and patients with “Unmet Needs”, which includes Pregnant Women, small children, diabetics, and hypertensive who suffer from nasal congestion, nasal drip, Inflammation, but cannot take any nasal sprays with steroids.
The “Unmet Needs” patients represents between 30 to 60 Million patients in America alone without a nasal spray they can use. In some cases, not having a nasal spray product can be life threatening. In China we have treated over 60,000 pregnant women, thousands of newborn children, and thousands of diabetic and hypertensive patients, while demonstrating both safety and efficacy, with no adverse events reported.
Two recently approved drugs for the treatment of Pulmonary Fibrosis have a Patient cost of approximately $100,000 per year. The market size for the treatment of Pulmonary Fibrosis is estimated to reach 4 Billion Dollars per year by 2023.